Our extensive network ensures you’ll be able to bridge the translational gap, no matter the area.
Our partner services
In vitro assays
Cytotoxicity (e.g. MTT, Trypan Blue)
Genotoxicity (e.g., MNT, Comet assay)
Apoptosis assays (e.g., Annexin V, TUNEL assays)
Mitochondrial toxicity
AMES testing
Customized assay development
In vivo assays
Toxicity testing (OECD 402, 411, 407, 408, 425)
Behavioural testing (e.g., T-maze, Morris Water Maze)
Motor function (Rotarod, Grip test)
Cognitive assessment (Touch screen, FOB, Operant testing)
In vivo efficacy and testing studies (ADME, PKPD)
Carcinogenicity studies
Animal Models (Transgenic, Inbred, Outbred, Syngeneic, Xenografted)
Customized animal studies
Bioanalytical
Metabolomics
ELISA
PCR
Western blotting
Flow cytometry
Next Generation Sequencing (NGS)
LC-MS/GC-MS
Ex vivo
Histopathological assessment (DAB, fluorescence)
Organ tissue sample collection
Immune profiling of tissue samples (cytokines, immune cells)
Slice-based electrophysiology
Consultation
Experimental Design
Risk management strategies
Project Management
Protocol Development
Quality Management Systems
Analysis
Statistical modelling (Linear modelling, ANOVA, etc.)
Pharmacokinetic parameter estimations (Cmax, terminal half-life, NCA, AUC)
Miscellanous
Biomanufacturing optimization (CDM optimization)
Antibody production (mABs, ADCs, BITEs, etc.)
Peptide manufacturing
Equipment Procurement
Workflow
We provide a seamless, full-service partnership to advance your preclinical research. Our proven workflow manages every step, from initial strategy to final report, ensuring quality.
We discuss your preclinical research needs during consultation and formulate a study proposal containing all the necessary experiments to be carried out.
The experiments are executed in confidentiality by vetted partner labs, with constant oversight by us, ensuring the project stays on track.
Once we obtain the experimental data, we perform and advise on analyses, compiling the final report for your review.
We provide long-term technical and scientific support for your projects, even after it has ended.
Scenario 1
Company T is developing a new immunotherapy against pancreatic cancer, and has just established proof-of-concept efficacy in vitro for their immunotherapy. Company T consults with Vivogenia on next steps for bringing their therapy forward. From the consultation, Vivogenia determines that Company T would like to perform a pharmacokinetic study to evaluate the persistence and biodistribution of their particular immunotherapy.
Study Design
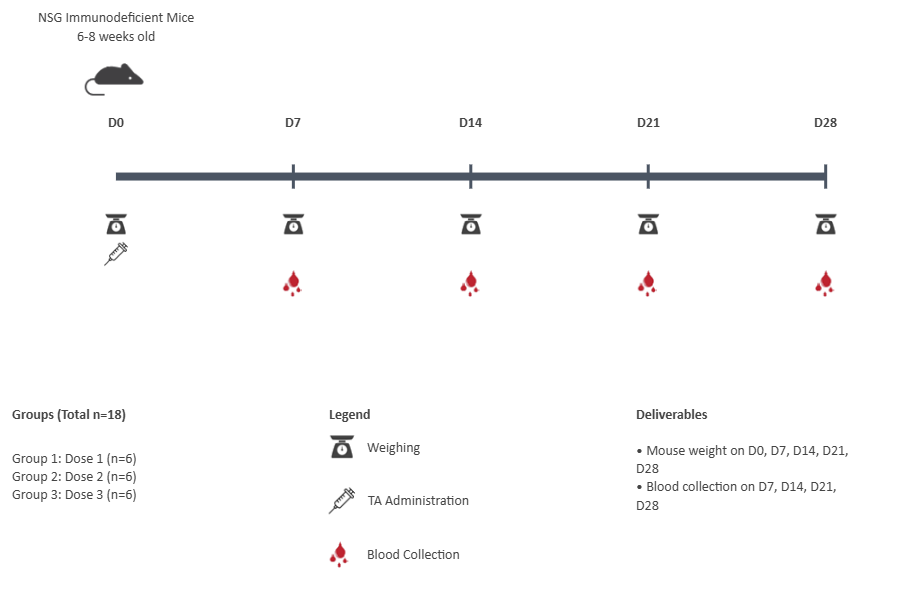
Animal model: NSG Immunodeficient mice, 6-8 weeks old
Dosing regime: Single injection, intravenous, 3 doses
Number of animals: 6 per dose
Study Details
Weekly sampling of blood based on predicted half life of similar class of therapeutic.
Weighing of animals for adverse immunotoxic effects from the doses.
Evaluation of the presence of test article (TA) in blood at different doses to assess
PK non-linearity.ELISA-based approach to the quantification of terminal half-life, AUC, Cmax and other
metrics of the test article.
Study Deliverables
PK data containing terminal half life, AUC, Cmax, Tmax derived from ELISA-based assay
Evaluation of non-linearity based on elimination rate
Report containing the details of the experiments.
Scenario 2
Company B is planning to register their health supplement product for regulatory approval in Australia. Company B consults with Vivogenia on the experimental data requirements needed to demonstrate their product is safe and efficacious. Vivogenia proposes cytotoxicity, genotoxicity testing for Company B, based on OECD guidelines for safety. Vivogenia also proposes in vivo bioavailability testing using mice models to understand the biodistribution of this product.
Study Design
Animal model: NSG Immunodeficient mice, 6-8 weeks old
Dosing regime: Single injection, intravenous, 3 doses
Number of animals: 6 per dose
Study Details
Weekly sampling of blood based on predicted half life of similar class of therapeutic.
Weighing of animals for adverse immunotoxic effects from the doses.
Evaluation of the presence of test article (TA) in blood at different doses to assess
PK non-linearity.ELISA-based approach to the quantification of terminal half-life, AUC, Cmax and other
metrics of the test article.